

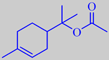
alpha-terpinyl acetate
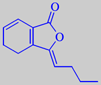
ligustilide
Levisticum officinale (Umbelliferae)
Lovage is a stout perennial umbellifer 1-2 metres heigh, probably originating in Persia. Already in the Middle Ages
it was a well known medicinal plant and spicy herb all over Europe. The leaves are used for flavoring soup, with new potatoes, etc.
The main volatile constituent of the leaves is alpha-terpinyl acetate, but the characteristic, refined, celery-like odour is due to a group of lactones called phthalides, with 3-butylidene-4,5-dihydrophthalide, or ligustilide, as a major component [6].
Lovage root oil is produced on a limited scale from the comminuted roots (photo on the right). It is used for special effects in perfumery and has one of the most penetrating odours of all natural materials.



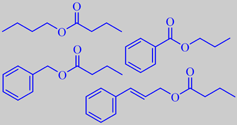
esters from mabolo fruit
Mabolo
Diospyros blancoi (Ebenaceae) Velvet Apple, Kamagong
Several species of the genus Diospyros of the ebony family have
edible fruits, all of them with persistent
four-fold sepals. Maybe the best known is the sweet and mildly flavored
persimmon, D. kaki, which is grown in large amounts in the subtropics.
The less known mabolo or velvet apple, D. blancoi, is a close
relative and a more aromatic so. A somewhat cheese-like odor is given
off from the velvety skin but is absent in the soft white flesh which
has a rich banana-apple-like flavor.
Pino et al. analyzed the volatiles from the fruit pulp of mabolos grown
in Cuba and found them to be characterized by many esters, particularly
benzyl butyrate (33.9 %), butyl butyrate (12.5 %) and (E)-cinnamyl butyrate
(6.8 %) [224]. Likewise Collins and Halim found a predominance of esters,
among them propyl benzoate [225].
Etymology: Gr. dios, heavenly, Gr. puros, fruit.
P.S. True ebony wood comes from Diospyros ebenum from southern
India and Sri Lanka. The almost black, heavy and extremely hard wood is
used, e.g., for fine musical instrument parts like this violin fingerboard,
tailpiece and chinrest.




odorants from Stephanotis headspace
Stephanotis floribunda (Asclepiadaceae)
In spite of its popular name, this plant has nothing to do with jasmine. It is a twining, evergreen, tropical bush of the family Asclepiadaceae with opposite, leathery leaves and white, waxy-looking, fragrant flowers.
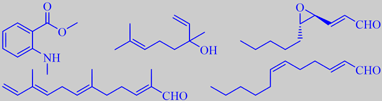
Methyl benzoate, methyl salicylate, 1-nitro-2-phenylethane, eugenol and the tertiary ocimenol 3,7-dimethyl-1,3(E),5(E)-octatriene-7-ol, also known from hyacinth, are important ingredients of the fragrance [5] [8] [69].
Etymology: Gr. Asklepios, the god of the art of medicine.



(+)-verbenone, isopinocamphone, (Z)-jasmone
Magnolia grandiflora (Magnoliaceae) Southern magnolia
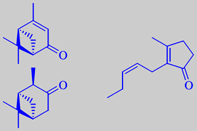
Southern magnolia is a native of the sandy river-banks in Florida, Carolina and Texas. It is a magnificent tree with dark-green, shiny leaves and huge, cream-coloured, delightfully fragrant flowers. In the Cretaceous Period this species inhabited Europe and Greenland, but vanished as the climate got colder. Now it thrives in the Mediterranean countries, blooming in July and August. The pictures shown are, from left to right, the flower bud, measuring up to 20 cm, the flower with its characteristic stamens, and the ripe seeds. Many members of the magnolia family have fragrant flowers, bark or wood - the famous Indian champak, Michelia champaca, should be mentioned.
Headspace-GC-MS analysis has revealed that the odour from live flowers of southern magnolia is dominated by three compounds: verbenone (18 %), isopinocamphone (9 %), and (Z)-jasmone (27 %). The two terpenic ketones alone have a camphoraceous odour. Amazingly, they combine with jasmone to create a beautiful and harmonic floral fragrance [5].
Etymology: The magnolias are named after the director of the botanical garden in Montpellier, Pierre Magnol (1699-1715).


methyl N-methyl-anthranilate, linalool, trans-4,5-epoxy-(E)-2-decenal,
alpha-sinensal and (E,Z)-2,6-dodecadienal
Citrus reticulata (C. nobilis) (Rutaceae)
The mandarin tree comes from southern China (a mandarin was actually a Chinese government official in a yellow dress). Today the mandarin fruit is mostly known as the seed-less, loosely peeled variety called clementine (photos), created by Pierre Clément in a lucky crossing experiment around 1900 when he was a leader of the agricultural school in Oran in Algeria.
Mandarin oil, made by cold pressing of the peels of (true) mandarin, has an elegant, deep, sweet, orange-like character, and is used in liqueurs and perfumery. The major odor impact compounds are the sesquiterpene aldehyde alpha-sinensal, also characterizing orange oil, and the aromatic ester methyl N-methylanthranilate, giving the oil a neroli-like character (and a blue fluorescence) [6]. Clementine peel oil, on the other hand, is dominated by unsaturated aliphatic aldehydes, e.g. trans-4,5-epoxy-(E)-2-decenal and (E,Z)-2,6-dodecadienal, with an odor reminiscent of coriander (leaf) and having a high tenacity on the skin, together with sinensal and linalool [212].




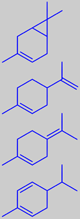
d-3-carene
limonene
terpinolene
a-phellandrene


important oxygenated aroma compounds in mango
Mangifera indica (Anacardiaceae)
The mango tree is one of the most important tropical fruit-trees. It is a native to South-East Asia and numbers some 30 species and a multitude of cultivars. M. indica is probably the best known species. Wild growing mangos are not known, but cultured mangos readily naturalize in tropical climates. Some lowland forests in the Hawaiian Islands are dominated by introduced mangos, for example. The young leaves are red-brown and slackly hanging down, a phenomenon often seen with tropical trees and probably an adaptation to heavy rain (first photo). The flowers have a mild and sweet odour. The taste of ripe mangos (M. indica) is sweet and aromatic and some varieties have a more or less pronounced turpentine-like shading - liked by some, disliked by others. Ripe mangos are quite juicy and messy to eat. However, those exported to temperate regions are, like most tropical fruit, picked under-ripe. Mango chutney, a popular accompaniment with rice tables, is made from unripe mangos. Dried unripe mango slices, 'aamchur', are used in Indian cookery (last photo - kindly supplied by Gernot Katzer). They are ground to a pale grey, sour and astringent powder with a slightly resinous overtone.
Pino and Mesa calculated the odour activity values of mango volatiles in 20 cultivars and found that the compounds potentially most important to mango aroma was ethyl 2-methylpropanoate, ethyl butanoate, (E,Z)-2,6-nonadienal, (E)-2-nonenal, methyl benzoate, (E)-beta-ionone, decanal and 2,5-dimethyl-4-methoxy-3[2H]-furanone (mesifurane) [173]. In a preceding study they found that the major volatiles were the terpene hydrocarbons delta-3-carene, limonene, terpinolene and alpha-phellandrene [174].