


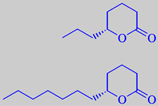
(R)-(+)-delta-octalactone and -dodecalactone
Cocos nucifera (Arecaceae)
The coconut palm probably originates in the Sunda Islands. It is one of the most important crops along tropic beaches around the world. The giant seeds of the coconut palm are spread by sea and have all they need for a long voyage. The outer leathery shell with fibres acts as a swimming belt, keeping the seed at the surface of the water. The coconut itself has a large energy reserve in its oily flesh, and about a quarter of a litre of water - the coconut milk. The coconuts germinate after being washed ashore (picture in the middle). In this way the coconut has colonized the beaches everywhere in the tropics.
The characteristic coconut aroma from the edible, white flesh (endosperm) of the seeds is mainly due to delta-lactones: (R)-(+)-delta-hexalactone, (R)-(+)-delta-octalactone, (R)-(+)-delta-decalactone and (R)-(+)-delta-dodecalactone [51]. Regarding lactones - see also peach.






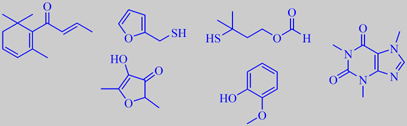
important aroma compounds in roasted, ground arabica coffee, and caffeine
Coffee
Coffea arabica (Rubiaceae) Arabica coffee
The coffee tree originates in the Ethiopian highlands. The red fruits
are containing two seeds, which, after being separated from the fruit
pulp, are known as 'green coffee'. Green coffee beans lack the colour
and characteristic aroma of roasted coffee; both are formed during the
roasting process.
Today's finest coffees are produced under strict quality control in all
steps from growing and harvesting to roasting and packaging the beans.
Connoisseurs consider perfectly brewed espresso to be the ultimate in
coffee because its special preparation amplifies and exhibits the inherent
characteristics of the beans.
The flavour of the coffee beans is developed by roasting at 185-240 °C
for about 12 minutes. Roasting is a pyrolytic process that greatly increases
the chemical complexity of the beans. Complicated chemical reactions take
place between sugars, proteins and minerals, e.g. by Maillard reaction,
Strecker degradation, breakdown of sulphur amino acids, breakdown of hydroxy-amino
acids, and degradation of pigments, yielding several classes of volatile
compounds: sulphur compounds, pyrazines, pyridines, pyrroles, oxazoles,
furans, aldehydes, ketones, phenols, and a large volume of carbon dioxide.
The caffeine content of the coffee beans is unaltered by the roasting
process and ends up at approximately 1.3 % in roasted arabica coffee.
About 900 volatile compounds have been identified. Based on odour activity
values, i.e. the ratio of concentration to odour threshold, the most important
aroma chemicals in roasted ground arabica coffee were determined to be
(E)-beta-damascenone, 2-furfurylthiol (or furfurylmercaptan), 3-mercapto-3-methylbutyl
formate, 2,5-dimethyl-4-hydroxy-3[2H]-furanone and guaiacol. However,
a shift in the concentration of the aroma components takes place from
roasted to brewed coffee because the polar compounds are preferentially
extracted. The sourness, bitterness and astringency of coffee is determined
by non-volatiles, including caffeine, which is responsible for the CNS
stimulating effect. As many of the important aroma compounds are sensitive
to oxidation, the shelf-life of roasted and ground coffee is short [15]
[16].
Etymology: Strangely, in Denmark coffee is called kaffe, whereas caffeine
is called koffein.
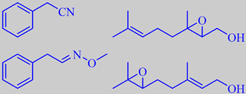



-2-decenalPlus.gif)
(E)-2-decenal and (E)-2-dodecenal
(+)-linalool
Coriandrum sativum (Umbelliferae)
The genus Coriandrum consists of this single species. Both the fresh leaves and the dried seeds are used, but their aromas are completely different.
The green leaves have a powerful and penetrating, 'aldehydic' aroma dominated by 2-alkenals, e.g. (E)-2-decenal and (E)-2-dodecenal [53] [54]. Used discretely, coriander leaves are brilliant in Oriental foods. For example, they are indispensable in Thai soup of the Tom Yum type - with coconut milk, lemongrass, ginger and chili.
The dried, powdered seeds, on the contrary, have a fine, sweet, flowery odour, primarily due to the tertiary monoterpene alcohol (+)-linalool [6]. The seeds have been found in ancient Egyptian tombs and in Pompeii.
Etymology: Lat. Coriandrum, from Gr. Koris, bed bug, as the aroma of the green parts of this plant may be reminiscent of the smell from the common bed bug, Cimex lectularius. Female bed bugs defend themselves during mating activities using a chemical spray of 2-hexenal and 2-octenal [311].


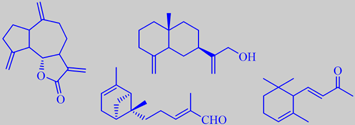
dehydrocostuslactone, costol, bergamotenal and alpha-ionone
Saussurea lappa (Compositae)
Costus root oil, a rare material, is steam distilled from the comminuted, dried roots of Saussurea lappa, a herbaceous perennial plant with yellow composite flowers growing in the Himalayan highlands, or by vacuum distillation of the resinoid extract obtained by extraction of the roots with solvents. It is a viscous, light yellow to brown liquid. According to Arctander, "it has a peculiar soft, but extremely tenacious odour, reminiscent of old precious wood, orris root, fatty (but not rancid) acids, vetiver, etc., with a distinctly animal or sebaceous undertone. The odour has been compared to that of human hair, fur coats or 'wet dogs'." Costus root oil has been used for intriguing notes in Oriental type perfumes.
Major components of the oil are sesquiterpenoid lactones, e.g. dehydrocostuslactone. Other components contributing to its characteristic odour are costol, bergamotenal and alpha-ionone [3] [55].
about new, artificial costus odorants
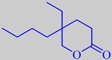
Costaulon ®, made by PFW Aroma Chemicals,
has a warm, intense, fatty, animalic odour, reminiscent of costus root
oil. Costaulon ® is chemically 5-butyl-5-ethyl-tetrahydro[2H]pyran-2-one
or gamma-butyl-gamma-ethyl-delta-valerolactone.


3-methyl-2-buten-1-thiol
2,5-dihydro-2,4,5-trimethylthiazoline
Fritillaria imperialis (Liliaceae) Kaiser's Crown
This stout bulbous plant of the lily family is native to an area stretching from eastern Turkey to the Himalayan foothills and is one of the earliest grown ornamental plants.
Still very popular in gardens, F. imperialis may be recognized by a characteristic foxy or skunky smell. One of the strongest smelling cultivars is the one called Premier, close to the wildtype.
It has recently been shown that the smell is due to 3-methyl-2-buten-1-thiol (or prenyl mercaptan) which is emitted from the whole plant and especially from the bulb [287]. Interestingly, the very same compound is responsible for the off-flavor formed in beer when it is exposed to light [288].
Etymology: Lat. fritillus, dice-box, i.e. the checkerboard pattern of the petals of another member from this genus,
F. meleagris.
p.s.
The characteristic odor of the red fox (Vulpes vulpes), however, is caused by another sulfur compound, namely 2,5-dihydro-2,4,5-trimethylthiazoline. This compound, excreted from the animal's anal glands, elicits fear and defensive behavioral responses in mouse and rat [289].



2(E),6(Z)-nonadienal,
'cucumber aldehyde'
Cucumis sativus (Cucurbitaceae)
The genus Cucumis includes 38 species, most of them from the arid zones of Africa. The cucumber was probably cultivated in northern India more than 3000 years ago. It was brought to the Mediterranean area, and already in Antiquity several sorts were grown. The melon is a close relative.
The characteristic, diffusive odour from cucumbers when they are sliced is mainly due to traces of the extremely powerful odorant 2(E),6(Z)-nonadienal, also called 'cucumber aldehyde', having an olfactory detection threshold concentration of about 0.01 ppb [56]. It is biochemically derived from unsaturated fatty acids, and is a typical example of what perfumers call a 'green' odour.