




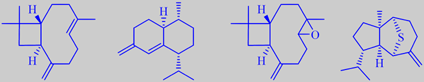
beta-caryophyllene, muuroladiene, caryophyllene oxide, and mint sulphide from Cedrela odorata
Cedrela odorata (Meliaceae) Cigar-box cedar
Cedrela odorata, a member of the Mahogany family (Meliaceae), is a deciduous tree from Central- and South America reaching a height of 30 m. In spite of its popular name it has nothing to do with true cedar, which is a conifer. Cedrela is the classical source of wood used for making cigar-boxes. Its red-brown wood is aromatic, light, soft and not attacked by insects.
The waste-wood from producing cedrela boards and veneer can be steam-distilled to yield an essential oil of pleasant dry-woody odour with an undertone like carrot seed oil. However, Cedrela odorata is now under protection as a threatened species in several countries.
Cedrela contains the main sesquiterpenes formed from cis- and trans-farnesyl pyrophosphate. Beta-caryophyllene and cis-4(14),5-muuroladiene have been found to be the major sesquiterpenes in the oil distilled from cedrela stems, and caryophyllene oxide the major oxygenated sesquiterpene [180]. The rare sesquiterpenoid mint sulphide has been detected in cedrela leaf essential oil [181].



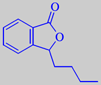
3-butylphthalide
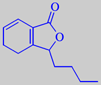
3-butyl-4,5-
dihydrophthalide
Apium graveolens (Umbelliferae)
Cultivated forms of celery all descend from the wild type, Apium graveolens, growing in saline areas near the coastline in Europe and western Asia. Celery, A. g. var. dulce has long, thick and crispy stalks. Celery root, or celeriac, A. g. var. rapaceum, has a swollen, beet-like root with a mild taste. Celery seed oil is produced in France, India and California. It is used chiefly for flavoring foods, vegetable juices, etc., although small quantities are also used in perfumery.
The characteristic celery flavour is mainly caused by the two lactones 3-butylphthalide and 3-butyl-4,5-dihydrophthalide (sedanenolide) [6]. See also lovage.



isobutyl angelate, 2-methylbutyl angelate, and chamazulene
Chamaemelum nobile (Anthemis nobilis) (Compositae)
Roman chamomile is a herbaceous perennial native to western Europe and North Africa. It is a close relative to the true chamomiles of genus Chamomilla (Matricaria), and the flowers, containing anti-inflammatory and sedative ingredients, have been used in much the same way.
Roman chamomile oil is produced from cultured varieties of the herb (var. flora plena syn. var. ligulosa) in several countries. The oil is obtained by steam distillation of the freshly dried flowers as a bluish liquid with a strong, aromatic odour characteristic of the flowers. Roman chamomile oil is especially suited for top notes in masculine perfumes, where it provides a heady, sweet-herbal diffusion of considerable radiance.
The major constituents of Roman chamomile oil are esters of angelic acid (2-methyl-2(Z)-butenoate) and tiglic acid (2-methyl-2(E)-butenoate), e.g. the isobutyl- and 2-methylbutyl esters. The bluish colour of the oil is due to the sesquiterpenoid chamazulene (C14H16) [6] [86]. The photo on the right shows the freshly distilled oil (dark blue) on top of the water phase.
Etymology: Gr. khamai, on the ground, low; Gr. melon, apple, because of the pleasant and somewhat apple-like odour of the plant; Lat. nobilis, noble.

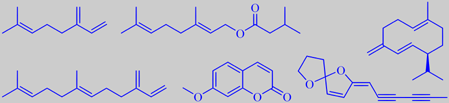
myrcene, geranyl isovalerate, germacrene D, (E)-beta-farnesene, herniarin and (Z)-en-yn-dicycloether
Matricaria discoidea (M. matricarioides) (Asteraceae/Compositae) Pineapple weed, Disc Mayweed
There are about fifty species of chamomile, more than half from South Africa and the rest from the northern hemisphere. Wild chamomile is from north-eastern Asia and western North America, but has now spread around the world. It is a "street weed" typically growing in newly cleared ground along roads and in courtyards. The tiny seeds are slimy in moist weather thereby sticking to feet and wheels.
The cone-shaped composite flowers without white ray-florets are aromatic, especially when squeezed, with a sweet herbal-fruity odor. The dried flowers may be used as a tea in the same way as true chamomile.
Lopes and Kolodziejczyk investigated the essential oil obtained by hydrodistillation from the aerial parts of M. discoidea grown in Alberta, Canada by GC/FID and GC/MS. Thirty-six compounds were identified, accounting for
87 % of the oil. The major constituents were myrcene (28 %), (E)-beta-farnesene (23 %), germacrene D (7%), geranyl isovalerate (6 %) and the so-called (Z)-en-yn-dicycloether (8 %) [215]. Moreover, 7-methoxy-coumarin (or herniarin) was detected in M. discoidea [216].
Etymology: Matricaria, from Lat. matrix, uterus, because chamomiles have been used against gynaecological disorders; discoidea, besause of the discoid flowers, chamomile, see Chamomile, Roman.



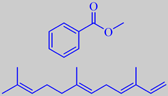
methyl benzoate and (E,E)-alpha-farnesene
from champak headspace
Champak
Michelia (Magnolia) champaca (Magnoliaceae)
Champaca
The legendary champak, a member of the magnolia
family, is a native of the temperate Himalayan region. However, it is
now found distributed throughout tropical and subtropical Asia. In India,
where the flowers are collected for perfumery purpose, it is found in
eastern Himalayas and eastern and western Ghats. The trees bloom once
during the monsoon and again in spring. When fully in bloom, the trees
are covered with thousands of golden yellow flowers with a powerful and
diffusive fragrance. The ripe seeds are seen in the photo in the middle.
Rout, Naik and Rao analysed the headspace from the live flowers by solid
phase micro-extraction. Methyl benzoate, phenethyl alcohol, phenylacetonitrile,
indole and methyl anthranilate, along with sesquiterpenes, e.g. (E,E)-alpha-farnesene,
constituted the body of the headspace. Moreover, ionones, e.g. dihydro-beta-ionone,
(Z)-methyl-epi-jasmonate, a number of aromatic esters, etc., have been
identified in extracts from the flowers [136].
Champak absolute is used in high-class perfumes where it may produce a
unique, warm, floral-leafy note which is often compared to that of a fine
grade of tea. The famous perfume Joy (Jean Patou 1935), a costly floral
scent, was based on champak absolute and other floral absolutes. Today
the champak flower volatiles are gently extracted using supercritical
CO2. The extract has an intensely sweet, floral-fruity odor with an apricot-like
undertone.
Etymology: Michelia, after the Florentine botanist Pietro Antonio
Micheli (1679–1737). Hindi Tchampaca.


matsutake alcohol, champignol
1-octen-3-ol, (3R)-
Champignon
Agaricus campestris (Agaricaceae) Meadow mushroom
The pleasant, characteristic flavor of a freshly cut champignon is dominated
by an alcohol called matsutake alcohol, because it was first identified
in the Japanese matsutake mushroom, Tricholoma matsutake (Tricholomataceae).
It is found in several mushrooms, and it is one of the odor components
from mould. Matsutake
alcohol, also called champignol, is chemically 1-octen-3-ol [6]. The naturally
occurring isomer is the (3R-) or (-)-enantiomer. This compound is available
from Bedoukian Research, Inc.
and is described as having a powerful, genuine mushroom odor with a slight
fruity note, less earthy than the racemic mixture.


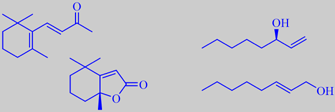
beta-ionone and dihydroactinidiolide, (-)-1-octen-3-ol and (E)-2-octen-1-ol
Cantharellus cibarius (Cantharellaceae) Golden chanterelle
The delicious and fleshy golden chanterelle, one of the most valued edible mushrooms, is widespread in temperate zones in acidic soil in deciduous and coniferous forests as well as in heather bushlands. It has an aromatic flavour often described as apricot-like and a slightly "hot" or peppery taste when raw (hence its German name "Pfifferling"). Golden chanterelle is among the richest sources of vitamin D known, with ergocalciferol (vitamin D2) as high as 2500 IU/100 grams fresh weight [258].
The slightly floral-fruity aroma of golden chanterelle is due to small amounts of carotene-derived compounds like beta-ionone and dihydroactinidiolide being present together with much larger quantities of 1-octen-3-ol and (E)-2-octen-1-ol. The latter compounds are characteristic of many mushrooms, e.g. champignon. [259] [260].
Etymology: Gr. kantharos, drinking-cup, Lat. cibarius, nutritional.
Golden chanterelle is mycorrhiza-forming and has been impossible to culture until lately. However, in 1997 Danell and Camacho reported the first successful fruit-body formation in the greenhouse, hosted by pine seedlings only 16 months old [261]. Since then the market prize has declined.
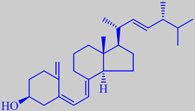
ergocalciferol, vitamin D2



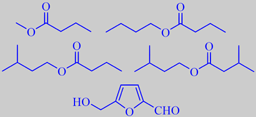
major cherimoya aroma volatiles
Annona cherimola (Annonaceae) Cherimola, Custard Apple
The tasty fruits of several Annona species are much appreciated in the tropics and subtropics. A. cherimola from the mountains of Peru was already cultured by the Indians in prehistoric times. Today it is also grown in the Mediterranean Countries, for example. The round, green and somewhat cone-like fruits are ripe when their skin gives slightly to pressure. They have creamy, white, richly sweet flesh with hard, dark-brown, glossy seeds embedded in it. The aroma depends on variety but can generally be described as being pineapple-strawberry-banana-like.
Ferreira et al. analyzed the volatile fraction in the fruits of four cherimoya cultivars and found that the major compounds were methyl butanoate, butyl butanoate, 3-methylbutyl butanoate, 3-methylbutyl 3-methylbutanoate and 5-hydroxymethyl-2-furfural [332].
Etymology: Quechua, chirimuya, cold seeds, because the tree grows at high altitudes i Peru.