
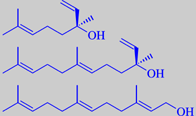
(+)-linalool, (+)-(E)-nerolidol and (E)(E)-farnesol
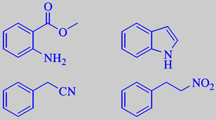
methyl anthranilate, indole,
phenylacetonitrile and 1-nitro-2-phenylethane
Neroli (Orange flower)
Citrus aurantium ssp. aurantium (Rutaceae) Bitter orange,
Seville orange
The flowers of various Citrus species are the source of the famous
neroli oils used in fine perfumery, particularly in Colognes. One example
is the flowers of the bitter orange, mainly cultured in North Africa.
Neroli oil is obtained by careful steam distillation of the freshly picked
flowers in about 0.1 % yield. An even more valuable material is the so-called
orange flower absolute, obtained by extraction (see boronia
for details on absolutes).
In both products, linalool is the main volatile constituent. Nerolidol
and farnesol are characteristic ingredients. Moreover, a number of N-containing
constituents are odour-determining, e.g. methyl anthranilate, indole,
phenylacetonitrile and 1-nitro-2-phenylethane [3] [6].
Etymology: It. neroli, from the French-born Anna Maria de la
Trémoille (1670-1722), princess of Nerola, a village near Rome.
She loved the fragrance of the orange flowers, and was the first person
to have them distilled. The tertiary sesquiterpene alcohol 3,7,11-trimethyl-1,6,10-dodecatriene-3-ol
was first identified in neroli oil, whence the name nerolidol. Somewhat
later, the isomeric alcohol 3,7,11-trimethyl-2,6,10-dodecatriene-1-ol
was also identified. It was correspondingly named farnesol, from Villa
Farnese, another village near Rome. Eau de Colognes have been created
since the end of the 17'th century. The classical ingredients were neroli
oil, bergamot oil,
orange oil,
lavender oil and
rosemary
oil. Colognes are still going strong, a modern example is Baldessarini
(Hugo Boss 2002).


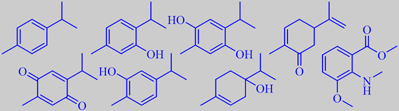
p-cymene, thymol, thymohydroquinone, carvone
thymoquinone, carvacrol, 1-terpinen-4-ol, and damascenin
Nigella sativa (Ranunculaceae)
The name Nigella, derived from Latin niger, means something like 'small black ones'. Carl von Linné thereby wanted to draw attention to the black seeds of this genus.
N. sativa is an annual herb native of southwest Asia. The seeds have been used for centuries as a medicine and as a spice . An Arab proverb calls them "the medicine for every disease except death." The variety of naan bread called Peshawari naan is usually topped with nigella seeds.
The seeds contains an essential oil of thyme-oregano-like aroma. A recent Algerian study found the main constituents to be p-cymene, thymol, thymohydroquinone, carvone, thymoquinone, carvacrol, and 1-terpinen-4-ol, together representing more than 36 % of the oil [186]. Moreover, the compound damascenin, or methyl 3-methoxy-2-methylaminobenzoate, an odoriferous 'alkaloid' characteristic of several nigellas, is present [187].


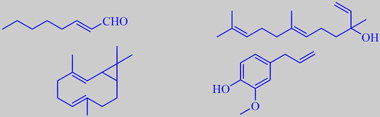
(E)-2-octenal, nerolidol, bicyclogermacrene and eugenol
Night-scented Stock
Matthiola longipetala (M. bicornis) (Cruciferae) Evening
Stock
There are about fifty species of the Cruciferan genus Matthiola,
having their main distribution from the Canary Islands, the Mediterranean
area, and into Central Asia.
Matthiola longipetala or Night-scented Stock is grown as an ornamental
primarily for its evening scent. It is a tenuous, low-growing and highly
branched crucifer with light purple flowers appearing wilted during the
day but awakening in the evening and then emitting a remarkably powerful
scent - strongly sweet and with a spicy nuance.
Hammami S et al. steam-distilled the fresh flowers of Tunisian-grown M.
longipetala and analysed the obtained oil by GC and GC-MS. Major
volatiles were (E)-2-octenal (2 %), nerolidol (2 %), bicyclogermacrene
(14 %), and eugenol (20 %) [318].
Etymology: Matthiola, from the Italian doctor and naturalist
Pietro Andrea Gregorio Mattioli (1501-1577), Lat. longipetala,
with long petals, Lat. bicornis, with two horns, i.e. the shape
of the fruit.



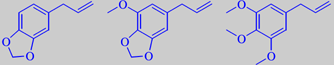
(+)-sabinene, (+)-1-terpinen-4-ol, safrole, myristicin and elemicin
Nutmeg
Myristica fragrans (Myristicaceae)
The nutmeg tree probably originates in New-Guinea. The fruit resembles
a smooth peach. When it ripens, it cleaves, and the purple-brown seed
with its lobed, coral-red coat becomes visible. The nutmeg as we know
it is the shelled kernel. Its use as a spice is due to an aromatic oil
consisting of approximately 90 % terpenes, with sabinene, alpha- and beta-pinene,
and 1-terpinen-4-ol as major components. However, a number of phenol ethers
play a decisive role for the overall fragrance, three of them are shown
[55]. Terpeneless nutmeg oil is sometimes used in masculine perfumes.
An elegant example is Cacharel Pour L’Homme (Cacharel 1981).
Etymology: Gr. myristikos, fragrant, Lat. fragrans,
fragrant. Nutmeg is rich in the fatty acid tetradecanoic acid, also called
myristic acid.


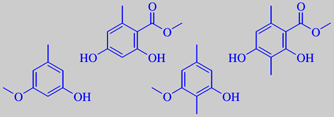
top: methyl 2,4-dihydroxy-6-methylbenzoate and -3,6-dimethylbenzoate
bottom : 3-methoxy-5-methylphenol and 3-methoxy-2,5-dimethylphenol
Oakmoss
Evernia prunastri (Usneaceae)
Oakmoss adds to the smell of the forest with its dry fungoid earthy-woody
odour. In spite of its name, oakmoss is not a moss but a lichen, growing
on the bark of deciduous and coniferous trees. It was used in perfumery
as early as the 16'th century. Baskets filled with it have been found
in the ancient royal tombs of Egypt, but whether it was intended for perfume
is not known. A mixture of phenolic acids extracted from oakmoss has been
used in drugs for treating external wounds and infections. In modern time
oakmoss has been collected in France, Morocco and Yugoslavia and extracted
for perfumery purposes ('Mousse de Chêne'), but today some of the
most important odour-active ingredients are made synthetically.
Oakmoss has the special odour notes associated with the warmth of the
fireplace, walnuts, the smoke from birch wood, etc. The key odorants are
the phenolic compounds shown above [3].
Oakmoss is used in countless perfumes of the fougère and chypre
types. For instance, it is evidently present in the dry-out notes of the
marvellous Kouros (Yves Saint Laurent 1981).
p.s.
The compound methyl 2,4-dihydroxy-3,6-dimethylbenzoate can be synthesized
in an interesting process from acyclic precursors (methyl crotonate and
methyl 3-oxopentanoate). It has an extremely penetrating odour, maybe
owing to quinoid tautomerism.
p.p.s.
For a true moss scent - see Liverwort.



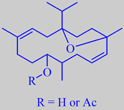
isoincensol and -acetate
Olibanum (Frankincense)
Boswellia carterii (Burseraceae)
Like myrrh
and opopanax, olibanum
is a gum-resin from small trees and thorny bushes of the Burseracean family.
They are growing in the northern Somali republic and in southern Arabia.
Olibanum is one of the oldest aromatic materials used by mankind. Already
in the Antiquity it was used as an incense in Mesopotamia, Egypt, Greek
and Rome. Ovid speaks of the solidified olibanum droplets as 'tears'.
The catholic church adopted the use of incense burning in the 5'th century.
A number of closely related Boswellia species exist. Several
publications deal with analysis of the volatiles from olibanum, but the
samples were often purchased from markets and so lacked a certified botanical
origin.
A recent study on a certified olibanum sample from B. carterii
identified alpha-pinene, beta-myrcene and limonene as the major monoterpenes.
Beta-caryophyllene, alpha-copaene, alpha-humulene and caryophyllene oxide
were the major sesquiterpenes. The characteristic olibanum compounds isoincensol
and isoincensyl acetate together with cembrene A were the main diterpenes.
Octyl acetate is a characteristic of B. papyrifera, but it was
not found in B. carterii [74].
The smell of olibanum smoke is influenced by a multitude of pyrolysis
products from the terpenoids present in the resin. Commercial olibanum
oil obtained by steam distillation of the resin from mixed Boswellia
species has a sweet, woody-balsamic odour with a fresh, almost citrusy
nuance. It is used in luxury perfumes, e.g. Opium (Yves Saint Laurent
1977).



(Z)-thiopropanal S-oxide,
the lachrymatory principle of onions
Onion
Allium cepa (Alliaceae or Amaryllidaceae)
There are about 300 species of Alliums, one from South Africa,
and most of the rest from temperate
Europe and North America. The ancestor of onion grows wild in Iran. Onion
has been cultured for more than 6000 years.
All Alliums are more ore less pungent from organic sulphur compounds.
The complex and labile onion flavour chemistry has been studied in detail
but will not be treated here. However, the lachrymatory (tear provoking)
effect of freshly cut onions, experienced by everyone, calls for a remark.
Amazingly, it was not until 1971 that the active principle was identified
as thiopropanal S-oxide (C3H6SO) [132]. When an onion is cut, alliinase
enzymes act on
S-alk(en)yl cysteine S-oxides giving sulfenic acids, which rearrange to
'the onion lachrymatory factor', now identified as mainly (Z)-thiopropanal
S-oxide [133]. The amount emitted varies among the different sorts of
onions, and also depends on the availability of sulphate nutrients in
the soil [134]. Schmidt et al. found that onion juice contains
1-22 micromol/ml thiopropanal S-oxide [135]. This volatile chemical is
water soluble, and exposure from it may be minimized by rinsing away the
onion sap with water.
Etymology: The name Allium was used by the Romans for garlic,
Allium sativum. Lat. caepe, onion.