

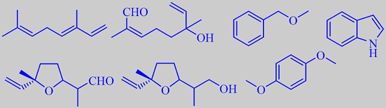
(E)-ocimene, 8-oxolinalool, benzyl methyl ether, indole,
lilac aldehyde, lilac alcohol and hydroquinone dimethyl ether
Lilac
Syringa vulgaris (Oleaceae)
Common lilac is a native of the northern Balkan Peninsula. Around 1560,
the German ambassador in Constantinople sent some specimens to Vienna,
and the culture quickly spread to the rest of Europe. Like jasmine, lilac
belongs to the olive family.
The beautiful fragrance of lilac flowers has recently been investigated
by modern headspace techniques, revealing (E)-ocimene to be the major
component, and the most characteristic components to be the furanoid terpene
aldehyde 'lilac aldehyde' and the corresponding alcohol (four diastereoisomers
each), benzyl methyl ether,
1,4-dimethoxybenzene (hydroquinone dimethyl ether) and indole. In fact
the diffusive aura of lilacs in full bloom is highly influenced by the
compound benzyl methyl ether. This simple but rare chemical has an intense
fruity-etheral odor, reminiscent of the top note in ylang-ylang
oil. Minor constituents of the lilac headspace are anisaldehyde, 8-oxolinalool,
cinnamic alcohol and elemicin [8] [66] [67].
Etymology: Gr. syrinx, tube or flute, because of the hollow,
marrow-filled branches; Lat. vulgaris, common.
P.S. As almost nothing is published on benzyl methyl ether, a note may
be relevant concerning the small-scale synthesis of an olfactory pure
quality of this odorant. Although the straightforward method would be
from benzyl chloride and a surplus of methanolic sodium methoxide, this
results in a product contaminated by an obnoxiously smelling impurity.
Instead, benzyl alcohol should be methylated in methanol under mild conditions
using an acidic catalyst and a water-removing agent (personal experiments).
P.P.S. The so-called syringaldehyde or syringic aldehyde, which is 4-hydroxy-3,5-dimethoxybenzaldehyde,
is derived from the glucoside syringin, first isolated from lilac bark.
Apparently it is of little importance in the headspace of lilacs.




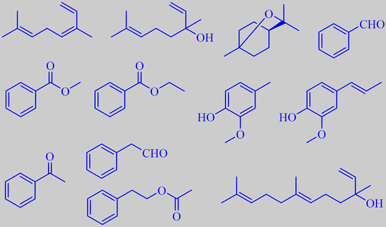
cis-ocimene, linalool, 1,8-cineole, benzaldehyde,
methyl benzoate; ethyl benzoate, creosol, isoeugenol,
acetophenone, phenylacetaldehyde, phenethyl acetate, nerolidol


Lilium orientalis (Liliaceae) cv. "Casa Blanca", "Sorbonne", "Yelloween"
Lilium longiflorum (Liliaceae)
The true lilies of genus Lilium include about 75 species. Multitudes of hybrids and cultivars exist and are highly valued ornamentals. Some are strongly scented to such a degree that cut flowers in an indoor vase may cause troubles to sensitive people. A recent European patent describes a method for reducing the emission from the flowers by adding certain simple enzyme inhibitors to the water. Headspace from the L. orientalis hybrids "Casa Blanca" and "Sorbonne", the L. orientalis trumpet hybrid "Yelloween", and L. longiflorum (photographs in this order) was analyzed by GC-MS:
Cis-ocimene and linalool were the major terpenoids in all varieties. Major compounds common to two or more varieties were: 1,8-cineole, benzaldehyde, methyl benzoate, ethyl benzoate, creosol and isoeugenol. "Sorbonne" had acetophenone as a special component, "Yelloween" was characterized by high amounts of phenylacetaldehyde and phenethyl acetate, and L. longiflorum by a high level of the sesquiterpene alcohol nerolidol [310].


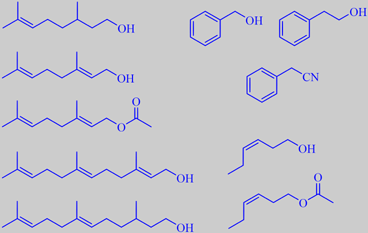
citronellol, geraniol, geranyl acetate, farnesol, 2,3-dihydrofarnesol
benzyl alcohol, phenethyl alcohol, phenylacetonitrile (benzyl cyanide)
(Z)-3-hexen-1-ol, (Z)-3-hexen-1-yl acetate

Lily-of-the-valley
Convallaria majalis (Liliaceae)
Lily-of-the-valley is a flower of the forest-floor on poor and calcareous
soil. In Scania in southern Sweden, for example, it is found in dense
stands close to the bed-rock. Lily-of-the-valley is widely distributed
in the northern hemisphere. The whole plant is poisonous.
The intense and elegant fragrance of the flowers may be described as "delicately
floral, with a distinct touch of greenness, surrounded by fresh, rosy-lemony
nuances" (Kraft et al.) [43].
In a study on the headspace of lily-of-the-valley flowers using GC-MS
and GC-sniffing/GC-olfactometry techniques, Brumke, Ritter and Schmaus
from the company Dragoco (today Symrise, Germany) identified some 23 compounds
contributing to the lily-of-the-valley fragrance, among these several
newly detected trace constituents [210]. The odorants could be divided
into floral-rosy-citrusy notes: citronellol (9.6 %), geraniol
(8.4%), nerol (1.3 %), citronellyl acetate (1.1 %), geranyl acetate (3.3
%), geranial + benzyl acetate (0.96 %), neral (0.02 %), benzyl acohol
(35 %), phenethyl alcohol (0.78 %), phenylacetonitrile (3.0 %), farnesol
(1.9 %) and 2,3-dihydrofarnesol (0.88 %), green-grassy notes:
(Z)-3-hexen-1-ol (11 %), (Z)-3-hexenyl acetate (7.8 %), (Z)-3-hexenal
(trace) and (E)-2-hexenal
(0.18 %), green pea and galbanum-like notes: 2-isopropyl-3-methoxypyrazine
(trace) and 2-isobutyl-3-methoxypyrazine (trace), fatty, waxy, aldehydic
notes: octanal (0.15 %), nonanal (0.1 %), decanal (0.07 %) and fruity,
raspberry notes: beta-ionone (trace). In another study of lily-of-the-valley,
phenylacetaldehyde oxime was identified [66].
Etymologi: Lat. convallis, valley; Lat. majalis, belonging
to May or blooming in May. In France lily-of-the-valley is called muguet.
Already in 1908 it was discovered that the compound 3,7-dimethyl-7-hydroxyoctanal or hydroxycitronellal has a beautiful lily-of-the-valley-like odour. This famous odorant is used in countless perfumes for this nuance. Unfortunately it is rather unstable and has shown up to be slightly allergenic, so its use level is restricted. Alcohols are more stable, and a number of successful alternatives have been developed, Mayol ® and Florol ® being examples [43].
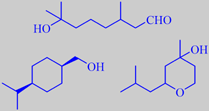
hydroxycitronellal
Mayol ® and Florol ®


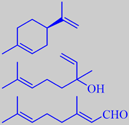
(+)-limonene, linalool, citral
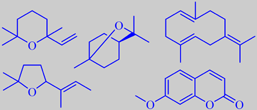
artifacts from distillation, and
germacrene B and 7-methoxycoumarin
Citrus aurantifolia (Rutaceae)
The little green lime fruit resembles lemon, but is more intense and aromatic. Yearly, more than 1000 tons of the so-called 'Distilled West Indian Lime Oil' (the main ingredient of Coca-Cola aroma!) is produced by steam distillation of the juice-and-oil emulsion from the chopped fruits. Lime oil produced this way contains a number of artifacts, compounds arising from the influence of acidic juice components upon the (+)-limonene, linalool and citral of the oil, for instance 1,8-cineole, 2,2,6-trimethyl-6-vinyltetrahydropyran and 2-(2-buten-2-yl)-5,5-dimethyltetrahydro-furan. These compounds contribute to the typical aroma of distilled lime oil [6].
Characteristic of lime fruit peel oil is the woody smelling germacrene B and the sweet smelling 7-methoxycoumarin [3] [68].


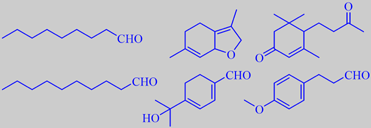
selected odorants from lindenblossom nectar
Lindenblossom
Tilia cordata (Tiliaceae) Litle-leaf Linden
Tilia platyphyllos (Tiliaceae) Large-leaf Linden
Tilia europaea (Tiliaceae) Linden
In Denmark, the lindens of the cities are mostly T. europaea,
a crossing between T. cordata and T. platyphyllos. In
southern Europe they are predominantly T. platyphyllos. However,
the separate species may be hard to distinguish.
The airy, honey-green fragrance from the large, blooming trees brings
a magic refreshment to the warm summer-breeze. Bees and other insects
love the nectar of the lindenblossoms, placed in the sepals of the hanging
flowers, protected from rain. The dried flowers make a delightful tea.
The chemistry of indenblossom fragrance is extremely complex. Naef et
al. recently made an interesting study of the change in volatiles taking
place by going from lindenflower (T. cordata) nectar via bee-stomach
to ripe lindenflower honey. The nectar volatiles were found to be composed
of fatty acid degradation products, e.g. nonanal and decanal, isoprenoids
like megastigmene-dione, a complex mixture of monoterpenoids, among them
the so-called 'linden ether' (2,4,5,7a-tetrahydro-3,6-dimethylbenzofuran)
and, tentatively identified as the major component,
8-hydroxy-p-mentha-1,3-dien-7-al. Moreover, a number of phenylpropanoids
were identified, among them
3-(4-methoxyphenyl)-propanal and the corresponding alcohol [27].




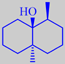
(-)-geosmin
-2-methyl-isoborneol.gif)
(-)-2-methyl-
isoborneol
Lophocolea heterophylla (Geocalycaceae) Variable-leaved Crestwort
The class of bryophyte (moss) plants called liverworts (Marchantiopsida) numbers 6000-8000 species! They are distributed globally in almost every available habitat, but most often in humid locations.
Liverworts are characterized by cellular oil bodies and often have distinctive smells when crushed, e.g. intensely sweet-woody, intensely turpentine-like, strong milk-like, sweet-mossy, fungal-like, carrot-like, mushroomy or seaweed-like scents.
Thus the crestworts (Geocalycaceae) typically have a strong and aromatic moldy (mossy) smell due to a mixture of (-)-geosmin, (-)-2-methyl-isoborneol and various sesquiterpenoids [275] [276] [277]. A common member of this family is the variable-leaved crestwort (photos) which is common in temperate zones on the base of trees, rotting log, fallen twigs and litter in woodland.
Etymology: Liverwort, from the cellular pattern of the thallus of genus Conecephalum which is reminiscent of hepatic lobules.