
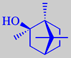
(-)-2-methyl-isoborneol
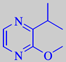
2-isopr.-3-methoxypyrazine
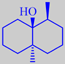
geosmin
1-octene-3-ol

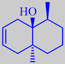
dehydrogeosmin
Mould
The microbial flora of mould depends on the biotope and the type of plant
material being degraded, but certain odour notes are general and may be
ascribed to specific secondary metabolites of the detritus organisms.
The following examples are typical:
2-Methylisoborneol has a strong camphoraceous odour, sometimes also recognizable
from ponds and lakes. You react reflexively if you get food into your
mouth having traces of this flavour - "it is mouldy"! 2-Methylisoborneol
is a metabolite of Streptomyces spp., Penicillium spp.
and Actinomyces spp. and has a very low olfactory detection threshold.
Geosmin has an odour known from moist cellars, and is also an aroma component
of beetroot.
It is a metabolite of Streptomyces spp. Actinomyces
spp. and blue-green algae.
2-Isopropyl-3-methoxypyrazine is another odour note from mould, reminiscent
of the odour of potato peel and green bell-pepper. It is a metabolite
of Penicillium spp., Pseudomonas spp. and Streptomyces
spp. [73].
1-Octene-3-ol is present in a multitude of mushrooms whose mycelium permeates
the soil. It is the most important aroma component in fresh champignons
[6].
P.S. Some cactus flowers, e.g. those of Rebutia marsoneri (Cactaceae),
shown on the right, give off dehydrogeosmin [5]. Presumably they are pollinated
by insects attracted by the smell of moisture.


muscone
muscopyridine
Musk
Moschus moschiferus (Moschidae), Musk deer
The small musk deer is living in valleys of high altitude in the Himalayas
and the so-called Tonkin Triangle. It is an unusual animal in a number
of ways. The males, for instance, haven't got antlers, instead they carry
an impressive pair of eye teeth in their upper jaw. Musk is the fatty
excretion produced by the males during the mating season in order to attract
females. The dried musk glands from killed animals have been sold at high
prices for thousands of years as one of perfumery's most precious materials.
Two of these 'musk pods' are shown in the photo on the right.
Today the musk deer is protected by conservational laws. Besides, muscone,
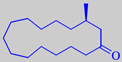
the major odorant from musk, can now be made by organic synthesis.
Natural muscone is (-)-(R)-3-methylcyclopentadecanone. It is found together
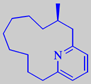
with small amounts of muscopyridine, supplying an urinaceous undertone
[3].
In several species of mammals the males use musks as pheromones. Other
examples are the civet
and the musk rat. The musk compounds
may be macrocyclic ketones as well as lactones. Musk lactones are also
found in some plants (see ambrette
seeds and angelica).
Both types have a deep, soft, musky character, but the ketones have the
most penetrating and animalic odour.
about new artificial musk odorants
Extensive research in the chemistry of musks for more than a century has
made a whole palette of macrocyclic musks available, some of them showing
new twists to the musk character. A few examples:
Velvione ® has a beautiful nitro-musk character (the nitro-musks were
the first artificial musks to be discovered; they had desirable olfactory
properties but are now largely banned for toxicological reasons). It is
said that the perfume Velviona (Helmut Lang 2001, limited release) contained
Velvione ® as the only ingredient!
Cosmone ®, newly released from Givaudan and the first C14-macrocyclic
musk commercially available, also has a nitro-musk character of great
warmth and diffusion. It plays an important role in the perfume Pi Neo
(Givenchy 2008).
Musk R1 ® (originally from Quest International) is an example of an
oxa-macrolide. It has a sensual, powdery musk character.
These musks are available from, e.g., The
Perfumer's Apprentice.
Macrocyclic musks are expensive odorants. Continued research therefore
aims at discovering new members with lower olfactory threshold values
(OTVs). It has been demonstrated that even thia-macrolides may have a
pleasant musk character.
7-Thia-pentadecanolide, for example, was shown to have an intense, linear
musk odor accompanied by green-mossy aspects and an OTV as low as 0.2
ng/l air [199].
Even non-macrocyclic compounds may show a musky character. Helvetolide
®, discovered by the Swiss company Firmenich in 1990, has a sophisticated,
modern musky note with a fruity pear aspect.
Etymology: Velvione, from velvet and ketone,
hinting at the velvety softness of the odor of this macrocyclic ketone.
Cosmone, following a tradition of grandiose naming of synthetic
musks, also alludes to the cosmetic appeal of this compound. Helvetolide,
lat. Helvetia for Switzerland.

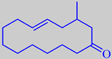
(Z)-5-cyclohexadecenone
Velvione ®
3-methyl-(E)-5-cyclotetradecen-1-one
Cosmone ®
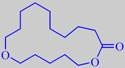
11-oxa-hexadecanolide
Musk R1 ®
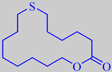
7-thia-pentadecanolide
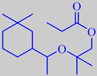
Helvetolide ®



cyclopentadecanone, Exaltone ®
Musk rat
Ondatra zibethicus (Muridae), Bisam
The musk rat is a native of North America. In 1905 three couples were
brought to Bohemia for the sake of the fur, some of their offspring escaped,
and soon the musk rat spread to most of Central Europe. I Denmark, the
musk rat has inhabited southern Jutland since the late 1990s.
The musk rat is a large semi-aquatic rodent. The eyes and ears are small,
and the hind feet are partially webbed. The tail is long, sparsely haired,
and laterally compressed. Musk rats live in freshwater and saltwater marshes,
swamps, and along the borders of ponds, lakes, and streams.
Musk rats possess a pair of musk glands which enlarge during the breeding
season, especially in males. These glands emit a yellowish secretion with
a strong musky odor. The odoriferous principles are some eleven macrocyclic
ketones with cyclopentadecanone (21 %) as one of the major constituents
[3].
In 1925 the Swiss company Firmenich started a production of cyclopentadecanone
for perfumery use (Exaltone ®),
based on the work on macrocyclic musks by Leopold Ruzicka (shown in the
laboratory on the photo in the middle). Ruzicka earned the Nobel Prize
in chemistry in 1939.
Pure cyclopentadecanone is a crystalline substance with mp. 63°C (photo).
It has a powerful and clean musk odor, very similar to that of muscone,
and, in the opinion of many perfumers, superior in type and beauty. It
ranks among the finest and most effective fixatives known, and it improves
the 'wearability' of a perfume when properly incorporated in the fragrance.
Its power becomes more perceptible and evident in extreme dilution, e.g.
below one percent in the perfume oil - according to Arctander (see referenced
literature).
Etymology: Lat. zibethicus, from Lat. zibetha, the name
of the musky smelling civet
cat.


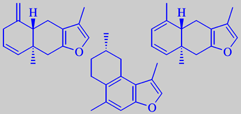
lindestrene (top left) and analogues
Commiphora myrrha (C. molmol) (Burseraceae)
Since the dawn of civilization, the southernmost countries on the Arabian peninsula, today Yemen and Oman, have exported myrrh (and olibanum) for incense ceremonies in the temples and principalities of the Orient. Myrrh is the dried gum-resin from a number of closely related, small, thorny trees of the genus Commiphora, probably originating in the highlands of Yemen (Wadi Hadramaut). Myrrh contains around 8 % essential oil with a refined, sweet-woody odour, especially noticeable when the gum-resin is heated in an incense burner. From this originates the Latin name 'perfumare' and the French 'parfum', meaning 'heavily smoking' or 'through the smoke'. Myrrh has antibacterial properties, it is affordable as a tincture in most pharmacies, and it was used for embalming in ancient Egypt.
The odour impact compounds in myrrh are a number of furanosesquiterpenoids, lindestrene being representative. The three shown compounds amount to 19 % of the essential oil [3].
Myrrh oil, obtained by steam distillation of the gum-resin, is used for 'Oriental' nuances in luxury perfumes, e.g. Burberrys for Men (Burberry 1981).




p-cresol

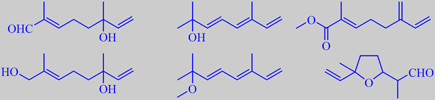
some terpenoids from narcissus flower extract
Narcissus
Narcissus poeticus (Amaryllidaceae) Poet's narcissus
Narcissus tazetta (Amaryllidaceae) Tazette
Narcissus jonquilla (Amaryllidaceae) Jonquil
The genus Narcissus includes around 25 species from the Mediterranean
countries, Central Europe and western Asia. They are recognised, for example,
by their bell-shaped corona.
N. poeticus, the narcissus praised by Homer, grows in sub-alpine
meadows from Spain to Yugoslavia with the southern Alps as its northern
border. Its corona has a crisp red margin (the first two photos). N.
tazetta, with several smaller flowers on its shaft, is common in
the Israeli mountains for example (the next two photos, taken in the Judean
hills west of Jerusalem). N. jonquilla has slender leaves and
2-6 brightly yellow flowers on its shaft. It grows naturally in the western
Mediterranean countries (last photo).
The odor of narcissus flowers has always fascinated perfumers - an elegant
and rich floral odor with a smooth animal undertone. There are lots of
cultivars, most of them are less fragrant, and for unknown reasons even
some with ugly filled flowers have been developed!
A number of well-known essential oil components like benzyl acetate, methyl
benzoate, p-cresol, phenethyl alcohol and indole are common to many narcissus
varieties, but none of these odorants are specific for narcissus.
Van Dort et al. analysed the extract of two fragrant varieties (N.
trevithian and N. geranium) derived from the above species
and found several new compounds. Some of these (from N. trevithian)
were synthesised in order to determine their odor, for example 8-oxolinalool,
3,7-dimethyl-1,3,5-octatriene-7-ol, methyl 2-methyl-6-methylene-2,7-octadienoate,
8-hydroxylinalool, 2-methoxy-2,6-dimethyl-3,5,7-octatriene and lilac aldehyde
- as shown above. However, no character impact compounds were identified
[189].
Etymology: Gr. narcosis, because of the 'narcotic' fragrance
of the flowers, or, Gr. Narcissos, a beautiful young man of Greek
mythology. It. tazzetta, small cup, because of the cup-shaped,
yellow corona of the flower. Sp. junquillo, 'little rush', i.e.
rush-leaved.