


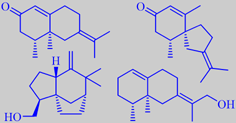
a-vetivone, ß-vetivone, khusimol, isovalencenol
Vetiveria zizanioides (Poaceae)
Vetiver oil is steam distilled from the aromatic roots of the tropical grass Vetiveria zizanioides, a robust grass as tall as a man and a native to the Indian subcontinent. Vetiver oil has a long history of use. Today, the main producers are Indonesia, Haiti and Réunion, but the grass is cultivated in many tropical and subtropical countries (the photo in the middle shows cleaned vetiver roots on sale for their fragrance).
Vetiver oil is a viscous amber-coloured oil with a characteristic rooty, precious-woody odour of great tenacity. It is olfactorily dominated by a complex mixture of oxygenated sesquiterpenes. The ketones alpha-vetivone (compare with nootkatone from grapefruit!) and beta-vetivone, which usually form more than 10 % of the oil, as well as khusimol and isovalencenol, 24-36 %, are the main constituents (data for Bourbon/Réunion oil) [146]. The identity of the character impact compounds is still disputed and vetiver oil is subject to continued investigations [43] [232] [233].
Vetiver oil is used in luxury perfumes for persistent green-woody notes. An even finer product, called vetiveryl acetate, is created by acetylating the sesquiterpene alcohols present in the oil. It has an elegant, soft, fruity-woody character. In classical perfumery, the oils of vetiver, patchouli and sandalwood in combination with a jasmine and gardenia complex was the base of the famous Crêpe de Chine note [3]. Vetiver products are used in many modern men's colognes, e.g Vetiver (Guerlain 1959), and the newer Hugo Dark Blue (Boss 1999) and Azzura (Azzaro 1999), and also (in lower percentage) in feminine compositions.
Lately, vetiver grass has gained focus for quite another reason. Its very excessive roots are effective in preventing soil erosion in the tropics where the original forest has been destroyed [231].
Etymology: Tamil, vetiver, root that is dug up, Lat. zizanioides, by the riverside (Linné), reflecting that the plant is often found along waterways in India. In perfumery the spellings vetivert or vetyver (French) are sometimes seen. In India the grass is more commonly known as khus.

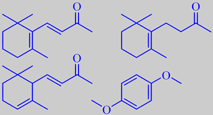
beta-ionone, beta-dihydroionone,
alpha-ionone and 1,4-dimethoxybenzene
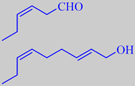
(Z)-3-hexenal and (E,Z)-2,6-nonadien-1-ol
Violet
Viola odorata (Violaceae) Sweet violet
Sweet violet is a native of the Mediterranean countries and Asia Minor.
From old ages it has been grown in gardens, and now it has spread to most
of Europe. It is flowering in early spring before foliation of the trees,
and it may form dense stands on the edges of forests, on slopes in parks,
etc., making itself known with the enchanting scent of the flowers.
In the vicinity of Hyères in southern France, cultured varieties
of V. odorata, called Parma violets and
Victoria violets, have been grown, not because of the flowers, but for
the sake of their green leaves. From these the expensive violet leaf oil
is obtained (around 0.1 %), with an odour character completely different
from that of the flowers.
The unique, fine, sweet fragrance of the violet flowers is dominated by
ionones: alpha-ionone, beta-ionone and beta-dihydroionone. Interestingly,
hydroquinone dimethyl ether or 1,4-dimethoxybenzene is another major constituent.
This compound has a powerful sweet herbal anisic odor which is barely
perceptible in the violet scent but acts as synergist to the ionones (at
least as perceived by human beings). Among the trace components a number
of secondary metabolites of linolic- and linolenic are important, e.g.
(Z)-3-hexenal with a powerful grassy
odor and (E,Z)-2,6-nonadienol with a powerful cucumber-like
odor [210]. These compounds are the prominent ones in violet leaf oil
which has a sparklingly intense 'green' odour being much appreciated in
fine perfumery [3].
The famous perfume Vera Violetta (Roger & Gallet 1892) showed the
first creative combination of natural violet leaf oil with synthetically
made alpha- and beta-ionone.
Etymology: Gr. ion, violet.
The perfumery-chemist Paul Bedoukian discovered in the 1960s during experiments with acetals of alkynals that 2-nonynal dimethyl acetal has a powerful odour resembling that of violet leaf oil. This compound is marketed by Bedoukian Research Inc. under the name of Parmavert ®.

2-nonynal dimethyl acetal, Parmavert ®







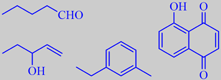
pentanal, 1-penten-3-ol and 1-ethyl-3-methyl-benzene from walnut flavor, and 5-hydroxy-1,4-naphthoquinone (juglone) from walnut husk
Juglans regia (Juglandaceae) Persian Walnut, Common Walnut
The walnut genus has about 20 species. Common walnut is a native of the region from Greece and the Balkan peninsula throughout southwest and central Asia and the Himalayas to southwest China. In Kyrgyzstan there are large walnut forests. The beautiful trees carry male flowers in drooping catkins, and female flowers in terminal clusters of two to five. The ripe fruit has a green, semi-fleshy husk around a hard, grooved shell surrounding the deeply folded seed. The related Pecan Nut, Carya illinoensis (Juglandaceae), is more oblong and has a smooth shell.
Walnuts are eaten as a delicacy all over the world, especially in salads (e.g. Waldorf salad), deserts and confectionery. They contain more than 50 % lipids with a high percentage of polyunsaturated fatty acids. It is well established that consumption of walnuts has benefits to health. For example, walnuts are high in omega-3-fatty acids and contain powerful antioxidants [138][139][140].
The high degree of unsaturation has an impact on the shelf-life of walnuts compared with other nuts, and is
behind the mild but characteristic walnut flavour (mostly perceived retronasally during eating). Tannins in the pellicle are responsible for the astringent taste as well as the brown color.
Elmore et al. analysed the headspace aroma compounds of walnuts from various locations and found that they were mainly lipid-derived aldehydes and alcohols, in particular hexanal, pentanal, 1-hexanol and 1-pentanol from linoleic acid breakdown, and 1-penten-3-ol from alpha-linolenic acid breakdown. Moreover, alkylbenzenes of molecular weight 120, in particular 1-ethyl-3-methylbenzene, were present. The proportions of these volatiles depended on the origin of the nuts [141].
Etymology: Germanic wal, foreign; Lat. jovis glans or juglans, Jupiter's nut; Lat. regia, royal.
P.S. Walnut leaves and husks contain a glycoside hydrolysing to a yellow compound called juglone, which is chemically 5-hydroxy-1,4-naphthoquinone. It is an allelopathic compound affecting the growth of other plants. Landscapers have long known that gardening underneath or near walnut trees can be difficult! Juglone finds use as a coloring agent in foods and cosmetics. It is also called Nucin.