

ectocarpene
allo-cis-1-(cycloheptadien-2',5'-yl)-butene-1
Brown algae
Ectocarpus siliculosus (Ectocarpaceae)
The olfactory impression of the fresh seashore is partly caused by algae
metabolites. Certain brown algae excrete a number of linear and alicyclic
C11-hydrocarbons as pheromones. The algae Ectocarpus siliculosus,
for example, emits an alkatriene named ectocarpene with a smell reminiscent
of tomato leaves [281].
about new artificial marine odorants
In perfumery, marine nuances are achieved using a methylbenzodioxepinone
named Calone 1951 ® (discovered by Pfizer in 1966). This unusual odorant
has a very intense 'sea-breeze' note with floral overtones and is the
basis of several perfumes of the marine trend, starting in the nineties.
L'eau d'Issey pour homme (I. Miyake 1994), Polo Sport Woman (R. Lauren
1996) and Aquawoman (Rochas 2002) are examples [43].
In a study of Calone homologues by Givaudan scientists, the derivate
7-(3-methylbutyl)-benzo[b][1,4]dioxepin-3-one turned out to be very interesting,
with an intense and diffusive, linear, marine odor, fresher and crisper
than Calone and with an odor threshold as low as 0.014 ng/L air [44].
A recent patent by Symrise describes 4-alkyl-substituted pyridines as
being very powerful marine odorants, e.g. 4-n-decylpyridine, which is
characterized by the valuable marine character prized and sought after
by perfumers, with additional ozone-like, buttery and orange-peel-like
nuances [282].
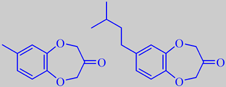
Calone 1951 ® and a new experimental homologue
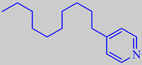
4-n-decylpyridine



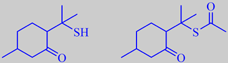
the strongly odoriferous 'sulphur-terpenoids'
from buchu leaf
Buchu leaf
Agathosma betulina, A. crenulata (Rutaceae)
The buchu has a restricted natural distribution area in the Western Cape
province in South Africa where it is found on sandy mountain slopes near
Niewoudtville, Piketberg and Tulbagh and in the Cederberg Mountains. It
is an evergreen, multi-stemmed, fragrant shrub growing to a height of
2 m. The buchu leaves have multitudes of round oil glands and are collected
for pharmacological and flavour/perfumery use. The Hottentots used them
for perfuming their bodies. However, the Cape Government now exercises
strict control over the gathering of buchu leaves and has lately made
the terms and conditions more onerous. In order to prevent the wholesale
destruction of the wild plants, no person is permitted to pick or buy
buchu without a licence [14]. The traditional pharmacological use encompasses
the treatment of kidney and urinary tract infections, colds, stomach ailments,
rheumatism, gout and fever. A. betulina is the more valuable
species as it has the lowest pulegone level, a compound considered to
be toxic.
Buchu leaf oil is obtained by steam distillation of the leaves as a dark
yellow to brown liquid with a characteristic, strong, minty-fruity odour,
reminiscent of black
currant. The major components of the oil are menthone and isomenthone,
diosphenol, limonene, pulegone and isopulegone. However, the constituents
responsible for the characteristic black currant odour are p-menthane-8-thiol-3-one
(mercapto-menthone) and its S-acetate (ca. 3 %). They are two examples
of the small number of naturally occurring sulphur-containing terpenoids
known to date [6][14].
Apart from its pharmacological use, buchu leaf oil is used as a flavour
ingredient (e.g., in fruit aromas) and in perfumery. The black currant
or 'Bourgeons de Cassis' note of buchu leaf oil is valued in certain types
of Chypre bases and Colognes. Only very small amounts are employed because
of the intensity of the oil.
Etymology: Gr. agathos, pleasant; Gr. osme, smell.
Bucku, the name used by the Hottentots.



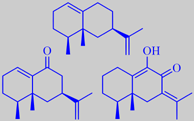
eremophilene, eremophilone, 2-hydroxy-e.
Buddawood
Eremophila mitchellii (Scrophulariaceae)
Buddawood, also known as Bastard Sandalwood or Desert Rosewood, is a small
tree common to the drier parts of South Australia, Queensland and New
South Wales - note the remarkably shaped trunk slice!
The wood contains an essential oil having an interesting and unique woody-peppery-spicy
base note. It works well as a fixative in fragrances and cosmetic products
and is now produced sustainably on a larger scale by Australian Botanical
Products [319] for use in modern perfumery.
The oil contains a number of sesquiterpenoids with eremophilene, eremophilone
and 2-hydroxy-eremophilone as major ingredients (the oil is thus biochemically
related to that of agarwood).
Etymology: Gr. eremos, desert, and phileo, love, i.e.
the members of this genus are adapted to arid environments. Lat. mitchellii,
named after the Scottish explorer of south-eastern Australia, Thomas Mitchell
(1792-1855).



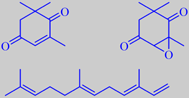
oxoisophorone, oxoisophoroneoxide and
(E)(E)-alpha-farnesene
Buddleja davidii (Buddlejaceae) Butterfly bush
Butterfly bush is native to north-western China. It is a deciduous to semi-evergreen shrub with a weeping form that can get up to 3 m tall and have a spread of almost 5 m. It has opposite lance-shaped, grey-green leaves on long arching stems. The flowers are borne in long cone-shaped clusters. In Denmark, the butterfly bush is restricted to gardens, whereas in southern England it is almost a weed, growing everywhere on abandoned sites, e.g. on top of crumbling walls. There are many garden cultivars with flowers of different colour - purple, white, pink or red - but they usually have an orange throat in the centre.
The flowers are some of the most attractive to butterflies (and nocturnal moths also). In northern Europe they are especially visited by the Nymphalides hatching in late summer like Vanessa atalanta, V. cardui, Inachis io (photo), Aglais urticae and, now and then, Polygonia c album.
To the human nose, the flowers have a peculiar velvety sweetness. Andersson et al. identified 41 compounds in the flower headspace with (E)(E)-alpha-farnesene (20 %), oxoisophorone (45 %), and oxoisophoroneoxide (10 %) as the major constituents [12]. Interestingly, these compounds also elicit strong antennal response in the same butterflies [13].



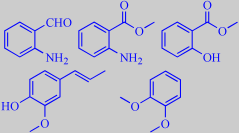
Bugbane flower volatiles
Actaea simplex (Cimicifuga simplex) (Ranunculaceae)
This herbaceous perennial has its native range in East Asian temperate regions - the photos are from Mount Ibuki in Japan. Several varieties are popular garden ornamentals, for example the dark-leaved variety called 'Brunette'. The flowers blooming in late autumn have a pronounced scent that could be described as sweet-spicy anthranilic-phenolic (from a chemist's perspective).
Groth, Bergström and Pellmyr analyzed the floral fragrance of A. simplex and found a number of benzenoid compounds confirming this, among them 2-aminobenzaldehyde, methyl anthranilate, methyl salicylate, isoeugenol and veratrole. A number of terpenoid compounds were also identified [358].
Extract from the root of the closely related A. racemosa is used medicinally for the relief of menopausal complaints. The active substances are various triterpen saponins as well as cimifugic acid and other phenol carboxylic acids.
Etymology: Bugbane, because the leaves are said to repel insects; Gr. Actaea, elder (Sambucus) because of a similarity of the leaves; Lat. simplex, unbranched.


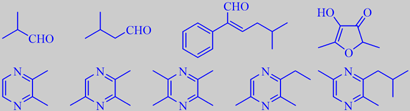
important aroma compounds in plain dark chocolate



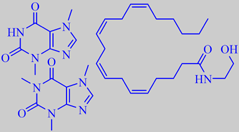
theobromine, caffeine and anandamide
Theobroma cacao (Sterculiaceae)
The cacao tree is a small underwood tree from the rainforests of tropical South America. It grows naturally in the low foothills of the Andes at elevations around 200-400 m in the Amazon and Orinoco river basins. The southern varieties are sometimes considered to be a species of their own, named T. leicocarpa. Today there are a multitude of cultivars and cacao is extensively grown in the tropics (West Indies, Java, Ghana, etc.).
The processing of cacao begins with a fermentation of the white pulp or mucilage covering the seeds. This fermentation is crucial, not only to the formation of significant volatile fractions (alcohols, esters and fatty acids), but also for the development of flavour precursors in the seeds (amino acids and reducing sugars). The thick pulp liquefies as it ferments and trickles away, leaving the seeds behind to be collected. The fermented and dried fatty seeds ready for export are now called cocoa.
By roasting the seeds, Maillard reactions converts flavour precursors formed during fermentation into three main classes of aroma compounds: aldehydes, O-heterocycles and pyrazines [123] [124]. The roasted seeds are then crushed and freed of their hulls and germs, and ground into a thick creamy paste called cocoa mass or cocoa liquor. Part of this is separated in cocoa powder and cocoa butter (last two photos).
For chocolate production, the cocoa mass is mixed with sugar, additional cocoa butter, emulsifier and, most often, vanilla or vanillin as an additional flavouring. A final process called conching (because of the shape of the original machinery) consists of grinding and aerating the liquefied chocolate for a prolonged period of time, thereby dehydrating and oxidizing the mass. This important step reduces tannins and improves the consistency and aroma of the chocolate [125].
The characteristic acidulous-bitter taste of dark chocolate is due to an interplay of tannins and theobromine plus a number of diketopiperazines, the latter being formed during the roasting process [126].
Cocoa butter is a unique vegetable fat in that it consists mainly of three triglycerides: palmitic-oleic-palmitic, palmitic-oleic-stearic and stearic-oleic-stearic. This composition of very uniform triglycerides provides a unique melting characteristic, making the chocolate melt slightly below 37 °C. Pure cocoa butter is used in white chocolate, and in various pharmaceutical and cosmetic applications.
Theobromine (1.5 - 3 % of the cocoa mass) and small amounts of caffeine are the CNS stimulants in cocoa [127]. The detection of arachidonoylethanolamine (anandamide), a brain constituent that binds to the cannabinoid receptor, in chocolate and cocoa powder has received particular attention [124].
Thanks to an exceptionally high procyanidin content, chocolate and cacao powder displays a high antioxidant activity [128].
Etymology: Gr. theos, god, Gr. broma, food, 'food of the gods'. Mixe-Zoquean cacao.



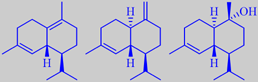
delta-cadinene, gamma-cadinene
and alpha-cadinol
Calendula officinalis (Asteraceae/Compositae) Pot marigold, Ringelblume, Maravilla
Calendula with its orange daisy-like flowers is known by every European. Being a native of the Mediterranean
area it is now used as an ornamental all over the world - in many varieties. The composite flowers open at sunrise and close during the afternoon but are generally short-lived. After a few days they give way to seeds, of which there are three kinds (heterocarpy). The outer curved and thorny seeds are shown above. Calendula has been cultured as a medicinal plant since the 12th century. It is particularly recommended for wound healing (as a diluted tincture made from the flowers) and is listed as GRAS (Generally Recognized as Safe) by the Food and Drug Administration, USA.
Handling of the flowers bring out a peculiar smell disliked by some people. The flower-stalks and sepals are covered by glandular hairs containing small amounts of an essential oil with delta-cadinene, gamma-cadinene and alpha-cadinol as the major ingredients [301] [302]. The disliked notes are probably caused by minor ingredients.
Etymology: Lat. Calendae, the first day of the month, or just month, as the flowers' openings and closings seem to mark the passing of the days; Lat. officinalis, used in medicine; cadinene, from cade juniper, Juniperus oxycedrus, in the oil of which cadinene isomers were first identified.